The Nexus of Open Science symposium took place on Nov. 14, bringing together leaders in neuroscience, clinical research, biomedical engineering and data science to explore topics ranging from FAIR data and software standards to improving the accessibility of AI tools in biomedical contexts like neuroimaging. Among the talks given, Georg Oeltzschner, Associate Professor of Radiology and Radiological Science at the Johns Hopkins School of Medicine, discussed a topic that may sound rather familiar to students with Organic Chemistry experience: Nuclear Magnetic Resonance (NMR) spectroscopy. To those without previous exposure, however, a NMR spectroscopy diagram may just look like a series of arbitrary peaks.
“Ultimately, because the protons in these compounds have characteristic chemical environments and their local electron density changes the magnetic field locally,” Oeltzschner explained, “each chemical environment has a different frequency, and these sets of frequencies are highly characteristic for different molecules.”
Outside dreaded organic chemistry midterms, NMR spectroscopy holds great utility in real-world applications, providing a non-invasive pathway to detect compounds in the human body, helping to diagnose and monitor the progression of diseases.
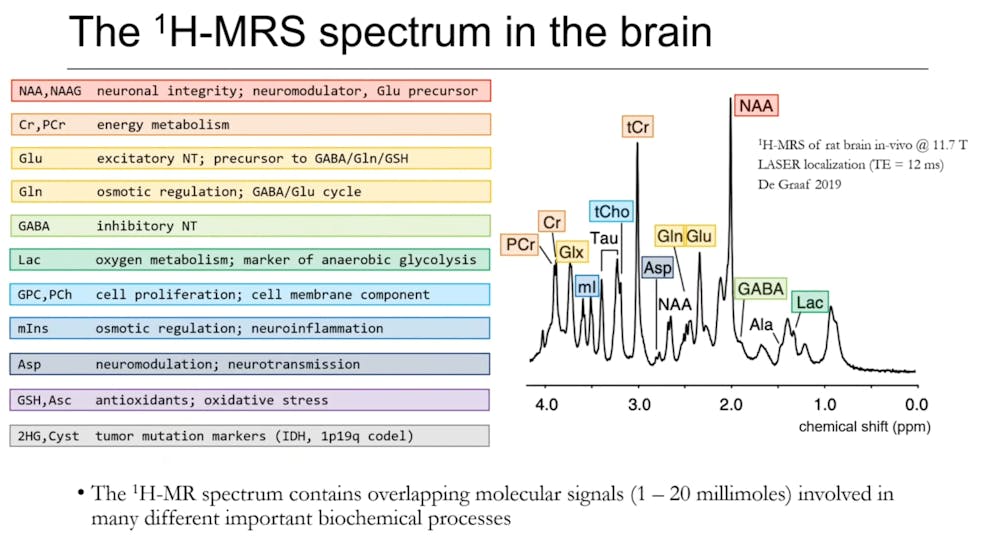
“In the human brain, we are able to differentiate about 20 molecules at about millimolar levels… We get markers of neuronal health, cell growth, neurotransmitters, antioxidants, markers of tumors and metabolites that are involved in energy metabolism,” Oeltzschner said.
One specific example of NMR spectroscopy’s utility in disease monitoring lies in the field of neuro-oncology, in which researchers are interested in tracking the effects of the tumor-suppressing drugs ivosidenib and vorasidenib on low-grade gliomas with the isocitrate dehydrogenase-1 (IDH1) mutation. This mutation is unique because it produces a compound called 2-hydroxyglutarate (2-HG), which interferes with normal gene expression, epigenetic regulation and cellular differentiation. The drawback to traditional imaging methods, such as magnetic resonance imaging (MRI), is that the effects these drugs have on tumor growth require months or even years to clearly manifest. This causes substantial delays in optimizing treatment regimens for patients in limited time. Meanwhile, magnetic resonance (MR) spectroscopy may serve as a viable alternative.
“If we do MR spectroscopy, we see a response to ivosidenib treatment within days to weeks,” Oetzlschner said. “The 2-HG signal disappears within days of treatment commencement… [Thus,] spectroscopy is a useful modality to quickly determine if the target engagement is actually working as intended. This is a piece of information that oncologists are very keen to obtain, especially because they cannot get it with any other imaging modality.”
Although MR spectroscopy holds great potential for imaging applications, it is important to acknowledge that MR spectroscopy diagrams themselves are not images. Unlike qualitative images, quantitative spectroscopy diagrams (spectra) are not intuitively interpretable and require extensive analysis – many organic chemistry students can testify to the difficulty of interpreting NMR spectra. Because spectra are not images, they are often not very compatible with an infrastructure of disease diagnosis and monitoring that heavily relies on images. A significant barrier to the advancement of spectroscopy in medical applications lies in the difficulty of reproducibility.
“The main problem is, we're looking at [super weak] signals… so this is a fight against noise at every turn… Ultimately… MR spectroscopy estimates of metabolite concentrations. But to get there from our raw data, we have all these [processing] steps that have to happen in between.”
There are a multitude of ways to process the spectra data and reduce noise, depending on the research or clinical context. Each differing decision, however, affects the end results and interpretation. The processing step of modeling also presents its own challenges.
“Why do we have to model spectroscopy data in the first place? Again, we want more than the qualitative approach… The [parameters] that we care most about are the amplitudes [signal integrations] that tell us how much of a particular metabolite is in our spectrum. These optimization problems tend to be ill defined; really difficult to solve,” Oeltzschner explained.
By the time the quantification step is completed, the same data may produce drastically different conclusions in the hands of separate labs. Consequently, the results become non-reproducible and regularization becomes necessary. For a long time, scientific research was held in the hands of an exclusive few, with high entry barriers for non-expert users, but that has gradually begun to change for the better in recent years. Oetzlschner and his collaborators have joined the fight for more open and equitable science by developing an open-source platform, Osprey, for processing and quantitative analyzing in-vivo magnetic resonance spectroscopy data.
“Osprey basically pulls all these modules into one automated workflow,” Oeltzschner explained. “No matter what scanner your data come from, it’s loaded and processed in a fully vendor-agnostic way. The tools to load and process these are all now out in the open for anyone to use. Anyone can read these data sets. There's no need to reinvent the wheel and write your own code for this anymore.”
Although versatile and streamlined, Osprey is just one step of a much larger scientific movement – one guided by a simple desire to share.
“We’re trying to continue this innovative spirit. None of these aforementioned tools [including Osprey] were explicitly funded, so this was largely a labor of love by the people who believed that the field needed change,” Oetzlschner emphasized. “We hope that we have made first steps towards bringing spectroscopy into the fold and making it more accessible to people who want to use it.”