The Department of Biology’s Fall 2025 Seminar Series opened with a packed house on Thursday, Sept. 11 as Simon Alberti, a professor in the Department of Cellular Biochemistry at the Technical University of Dresden, delivered a talk titled “Biomolecular condensates: molecular insights and implications for disease intervention.”
Alberti began by explaining ribonucleoprotein (RNP) granules – large, cellular structures containing RNA and proteins that rapidly assemble and dissolve in response to stress. Alberti’s research dissects the physical principles governing the formation of RNP granules. Central to this is the concept of phase separation, a cellular phenomenon in which certain proteins are able to aggregate and separate from the rest of the cytoplasm. The result is a highly dynamic condensate.
Alberti’s team knew that stress granules assemble when polysomes disassemble and mRNA is released, yet he acknowledged that this is still in theory to some extent. They set out to reconstitute these granules. In the absence of RNA, nothing really happens. But when they mixed in the RNA, they observed similar dynamics in cells. Adding more stress-related proteins showed that the composition of these stress granules depended primarily on protein-RNA interactions. This mattered because disease-related proteins like TDP-43 only join stress granules when they can bind RNA. Once inside, they clump together, which is a problem seen in neurodegenerative diseases like ALS.
They also investigated what the RNA is doing by including a short hairpin RNA with dyes at the end to track signals and measure folded or unfolded. The results were surprising.
“Outside the condensates, the hairpin was primarily folded, but inside the condensates, it was primarily unfolded. So there clearly is something happening inside the condensate,” Alberti said.
He went on to explain that G3BP, a key protein in stress granules, grabs single stranded RNA and stabilizes it, hence why it’s unfolded inside the condensate. But the story does not end there. When helicases and ATP were introduced, previously solid-like granules melted into dynamic liquid droplets.
“So this shows you how powerful these enzymes are and how they can change the properties of these condensates,” he said. These changes reveal how cells can adjust the state of stress granules, helping the cells survive and recover from sudden stress.
The functional significance of RNP granules has long been hypothesized to center on translational regulation; that is, deciding when and how much protein gets made from RNA in the cell. To test this, Alberti’s team measured the amount of translation of mRNAs in condensates and found that they were translated substantially less than free mRNAs, but mutants of G3BP1 that could not form condensates showed translation rates identical to controls.
“So this shows you very nicely that it is the sequestration of the RNA, not so much the binding, that leads to the repression of translation,” Alberti said.
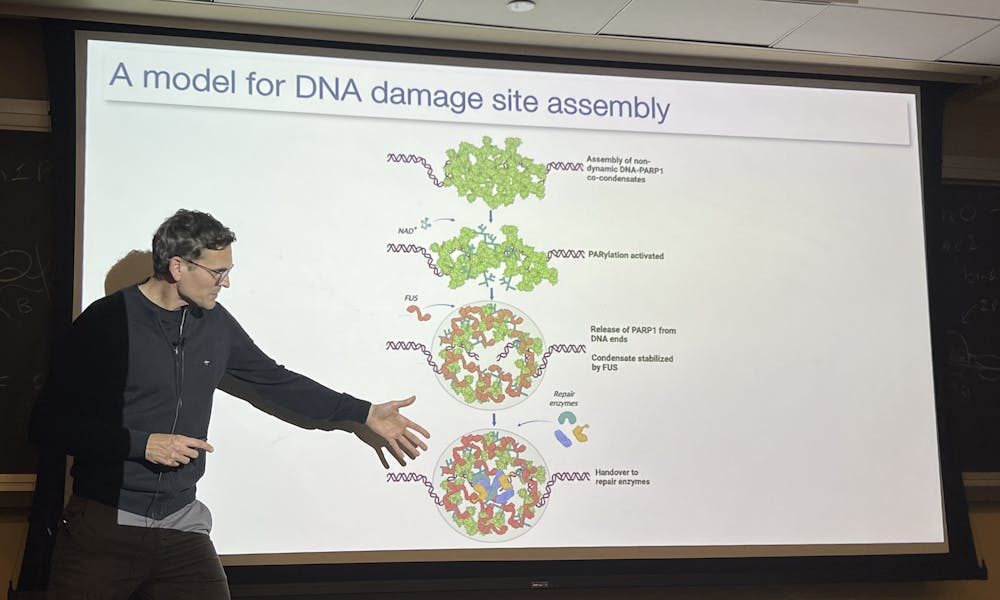
Alberti then addressed one of his research’s main focuses: the formation of condensates, and the role they play in DNA repair. DNA can be damaged for a variety of reasons, especially in cases like infections or radiation. When this happens, damage sites typically assemble, with various repair factors to support its reassembly.
He described various experimental techniques he used to investigate the molecular mechanisms involved.
“The many years of work was really like solving a puzzle,” he described. For example, to observe which proteins would be involved in DNA repair, a common series of experiments involves cutting DNA strands using a laser in vitro, and the arriving proteins would be noted.
The exact mechanism by which the two strands of DNA would hold together was mysterious, and so experiments were conducted where short DNA was mixed with the purified protein PARP. An interesting investigation involved fixing a single long DNA molecule to a surface and stretching it out. As seen in a recording shown by Alberti, the PARP protein “reeled in” the DNA, forming a condensate at the fixed end of the molecule. These experiments highlighted the role of PARP protein in detecting and repairing DNA damage.
More importantly, PARP played a key role in condensate formation. Alberti highlighted this experimentally in another case where long DNA was fixed at two ends with a restriction enzyme cut site in the middle. In the presence of PARP, a condensate would be formed at the cut site to hold it together, while in its absence, the DNA would be pulled back from both ends. Alberti further highlighted PARP’s significance for the condensation process through inducing mutations that lowered PARP recruitment. Far from restricting his focus to PARP, Alberti emphasized the significance of considering the roles of other elements, such as FUS, NAD+ and MRN complexes that may also have key interactions with the functions of condensates. When hypothesizing why condensates may be needed rather than just simple dimers, Alberti suggested they may provide additional mechanical resistance and stability holding damaged DNA together.
Alberti then highlighted the important applications of these experiments in the treatment and understanding of diseases. He drew attention to the current use of PARP1 inhibitors in FDA-approved treatments for some cancers, suggesting their effectiveness may be reliant on the formation of condensates. He described his team’s experimental work to compare the effectiveness of these drugs on stabilizing condensates.
Alberti highlighted that the mechanisms of condensate formation were not fully known, there was unwanted resistance in the drugs’ ability to stabilize condensates. He argued that if these mechanisms were better known, available therapies could improve. Diving deeper, he suggested that making PARP protein more dynamic could potentially resolve the development of resistance against these treatments, as it would preserve the effectiveness of the condensates they could form. Thus, Alberti ended the session by summarizing the talk and emphasizing the potential of further investigating these mechanisms.