On Monday, Nov. 27, postdoctoral fellow Kiara Eldred from the Thomas Reh Lab at the University of Washington gave a talk titled "Visualizing Progenitor Cell Trajectories in the Developing Human Retina" for the Department of Biology.
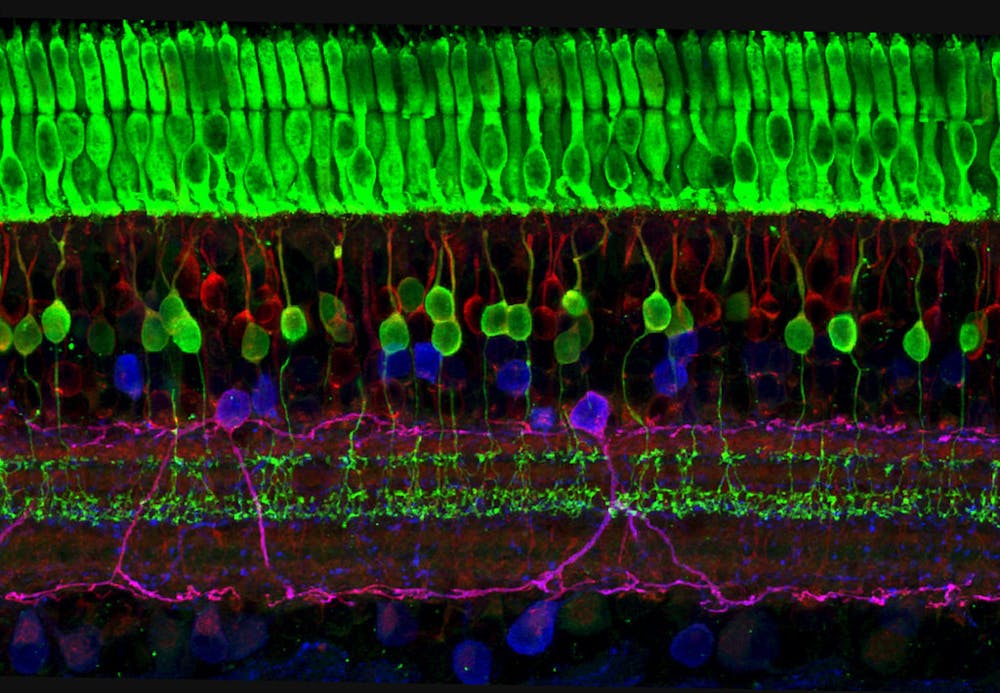
The retina is a layer of cells that captures incoming light particles (photons) and transmits them to the brain using electrical and chemical signals. The layer is composed of diverse cell types that are differentiated at distinct stages of development — in other words, some cell types form earlier than others. Which cell type is formed is determined by the pattern of gene expression in the cell.
Eldred’s research investigates how progenitor cells, descendants of stem cells that can further differentiate into more specialized cell types, in the retina determine the eventual fate. This can provide insight into the development of the human eye.
“If we can learn more about how these cells choose their fate in development, maybe we can use principles to regenerate retinal tissue,” Eldred said during the talk.
To explore how the regulation of gene expression determines the cell fate of retinal cells, Eldred mapped the presence of open chromatin regions. Genetic information is stored in a complex of proteins and DNA called chromatin. Open chromatin regions are regions that are less condensed, making them more accessible to regulatory mechanisms that facilitate transcription and translation of this part of the DNA. This allows genes to be expressed and relevant proteins to be produced. More open chromatin is correlated with heightened gene expression.
Eldred introduced a novel technique she developed called CUT&TIME, which uses 6-methyl adenosine methyltransferase (m6A) to bind to open chromatin regions. m6A was chosen because of its binding efficiency to open chromatin regions. Undifferentiated human-origin retinal cells were infected with a lentivirus that contains m6A. They were then allowed to grow and differentiate.
Eldred tested the methodology on retinal ganglion cells (RGCs) because they form relatively early during the retina development process. The change in the pattern of m6A markers would be tracked to look at how open chromatin regions changed as the progenitor cell differentiated into an RGC.
Eldred used two main considerations to verify the effectiveness of her methodology. Firstly, she found that open chromatin regions were primarily found at transcription start sites, indicating that differentiation was associated with the transcription of developmentally important genes. Eldred also found that the inclusion of m6A did not affect RNA polymerase II activity by comparing RNA polymerase binding in m6A marked genes and unmarked genes, meaning that m6A binding did not affect transcription.
“When we compared RNA polymerase binding, we get a very similar pattern on the start site... We’re not changing [polymerase II] activity,” Eldred said.
By allowing m6A to bind to open chromatin at different time points during a single cell’s development, Eldred visualized the developmental trajectories of progenitor cells as they eventually developed into RGCs using retinal organoids, which are induced from stem cells and can form into different retinal cell types. Eldred visualized the cells using uniform manifold approximation and projection (UMAP) that allowed the examination of data at the single-cell level. She suggested that the m6A markers could be used to distinguish between cells at different stages of development.
“This was a really exciting image for us to get because what it shows you is that we can discriminate between… retinal ganglion cells versus progenitors… so these m6A marks do differentiate cell type identities based on open chromatin profiles,” Eldred explained.
The two main implications of Eldred’s CUT&TIME methodology were its applications to tumorigenesis in retinoblastoma and regenerative treatment. Retinoblastoma is a tumor that starts in the retina and is the most common type of eye cancer in children. It is associated with mutations in the RB1 gene, which affects retinal cell development and causes tumorigenesis. By using the CUT&TIME methodology to track the developmental trajectory of retinal progenitor cells, researchers could gain insight into how the developmental pathway is affected in retinoblastoma.
Eldred then discussed how the results from her CUT&TIME technique could have applications in regenerative treatment. Eldred introduced Muller glia, stem cells that could regenerate and form bipolar cells and prevent vision loss, in other animals. These glia were localized in proliferative zones that were found even in adult animals. Eldred postulated the existence of a similar late proliferative zone (LPZ) in the developing human retina. Applying the CUT&TIME methodology to the LPZ could allow researchers to track cell differentiation of different cell types in a small concentrated region.
“In these other creatures, [the LPZ] is the last region of the retina to continue to proliferate… so that’s the first question I asked: Is this similar in human tissue?” Eldred explained.
Eldred hypothesized that the edge of the human retina could potentially be an LPZ and tested her prediction by examining cultured fetal retinal tissue. She investigated the expression of two genes, VSX2 and PAX6, whose co-expression would suggest the presence of early retinal progenitor cells. Eldred also performed ssRNA sequencing of this potential LPZ and compared results between fetal and adult retinal tissue. She found a decrease in less developed retinal cell progenitors in the region from the fetal LPZ to the peripheral retina and a corresponding decline in the expression of VSX2 & PAX6. This demonstrated that over time, the LPZ lost its regenerative capabilities because fewer retinal progenitor cells were seen in the region.
It was also found that there were RGCs (early-stage development cells) and bipolar cells (late-stage development cells) in the same small LPZ region. Eldred’s results demonstrated that the fetal LPZ had progenitor cells for retinal cells that formed both early and late in retinal development.
“We don’t see all the same progenitors in the same place — in the [central retina], we would see late progenitors, and in the periphery, you would see the early progenitors… so it’s exciting to see all of them in the same smaller region,” Eldred explained.
After explaining how she confirmed the existence of a fetal LPZ, Eldred ended the talk by discussing her current and future work. She hopes to understand the regulatory logic governing progenitor cells in the LPZ to understand when early and late-stage cells differentiate.
“I hope to use [CUT&TIME] to identify targeted therapies for regeneration to restore vision… I would like to use [the LPZ] to identify factors that modulate retinal proliferation,” Eldred concluded.