On Thursday, Oct. 12, Associate Professor in the Department of Molecular Biosciences at Northwestern University Sadie Wignall shared her findings on the mechanisms oocytes employ to regulate spindles without centrosomes during meiosis.
The seminar, titled “Stabilizing a Dynamic Structure: Mechanisms that Maintain Acentrosomal Spindle Integrity During Oocyte Meiosis,“ was hosted by the Department of Biology at Hopkins for their seminar series.
During cell division, macromolecule structures known as spindles form to segregate chromosomes. Centrosomes facilitate spindle assembly in most cells. Centrosomes nucleate microtubules, which make up the spindles, embedding slowly-growing minus ends of spindles into the centrosome and radiating dynamic, fast-growth plus ends outward to the rest of the cell.
During her postdoctoral fellowship, Wignall began studying meiosis — a type of cell division that produces reproductive cells with half the number of chromosomes — occurring in female reproductive cells or oocytes.
"What is special about these cells is they actually degrade their centrosomes, so the cells don't have them at all," she said during the seminar.
Although oocytes in humans and most model organisms exhibit acentrosomal spindles, the Wignall lab chose to use the nematode Caenorhabditis elegans (C. elegans) as their main model system because the transparent bodies of C. elegans allow for live imaging of these processes.
One of Wignall's early studies classified the stages of spindle assembly in C. elegans oocytes. They identified an early microtubule cage stage, composed of chromosomes surrounded by bundles of polymerizing microtubules constrained by a nuclear envelope that has begun to disassemble. As microtubules start to organize, their minus ends begin to come together and move outward away from the chromosomes, forming multiple spindle poles at the periphery of the array in what is called the multipolar stage. The spindle poles finally coalesce until there are just two, reaching the bipolar stage. This spindle assembly pathway is distinct from cells with centrosomes, where duplicated centrosomes nucleate microtubules, migrate to opposite ends of the cell and form spindle poles.
Wignall became interested in the factors that maintain spindle integrity after it was reported that even after spindles reach the bipolar stage, spindle poles still continue to split apart and coalesce. Although most restore bipolarity, spindles that exhibit this instability have a much higher chance of generating errors in chromosome segregation due to incorrect attachments to chromosomes.
By taking advantage of the auxin-inducible degron system, which enabled the depletion of tissue-specific proteins in a shorter time frame than conventional methods, Wignall could effectively pinpoint proteins necessary for stabilizing spindle structures.
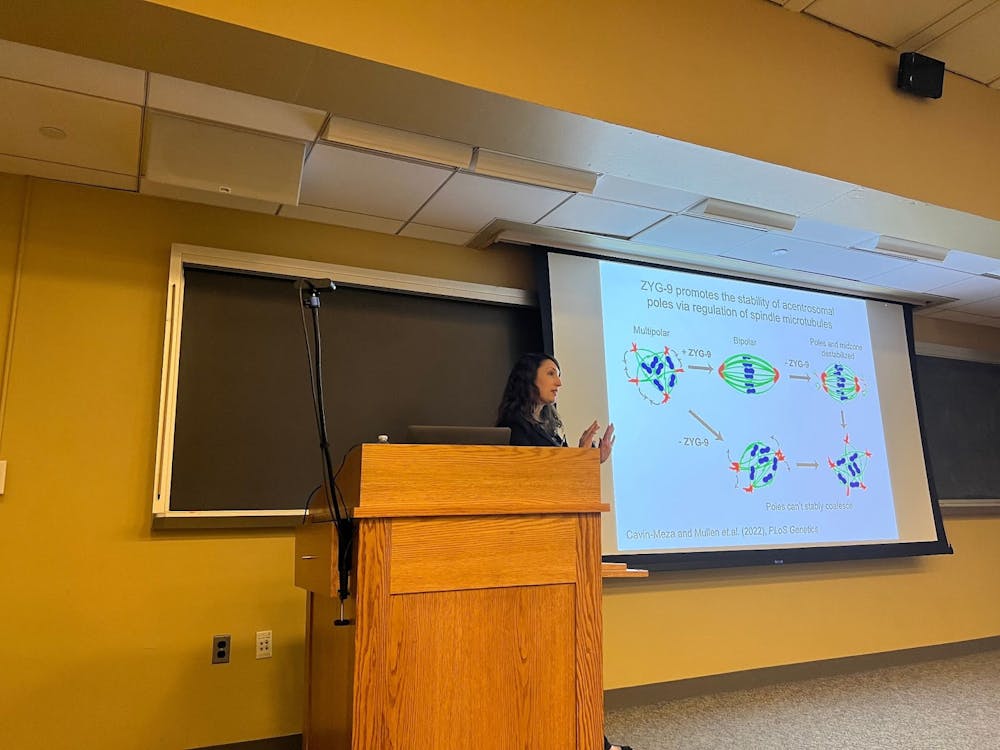
One protein found to be required to maintain the stability of acentrosomal spindle poles was ZYG-9.
"If you remove ZYG-9 before spindle assembly, the poles can't properly coalesce, and you just get a multipolar spindle," Wignall explained. "If you remove ZYG-9 from a pre-formed spindle, this destabilizes both the center of the spindle and the poles. Eventually, the spindle can't maintain its integrity and falls apart."
Wignall also uncovered proteins that help spindles achieve a balance in force. Among the proteins was KLP-18, a motor protein that was found to provide a force that pushes the minus ends of spindles outward. When KLP-18 is depleted, the absence of the outward force causes spindle poles to coalesce into a single pole, forming a monopolar spindle structure.
"[This finding] shows us that there is some sort of inward force that pulls spindle poles together rapidly when you inactivate KLP-18," she pointed out.
Wignall found that dynein was crucial in exerting this counterbalancing inward force and limiting spindle sizes, a finding that was consistent with that in mitosis.
An observation that surprised Wignall was that when chromosomes detached from microtubules, these microtubules would reorganize around the chromosomes and later segregate them, even in the absence of both KLP-18 and dynein. This led her to the eventual revelation that BMK-1 was providing a backup outward force that led to spindle reorganization for segregation.
Another major question that Wignall set out to answer was how chromosomes move on spindles. In spermatocytes, chromosomes have kinetochore proteins that make end-on attachments to the spindle. In oocytes, they still utilize kinetochore proteins, but spindles run alongside the chromosomes, forming lateral attachments.
Wignall found that a motor protein, KLP-19, forms in the center of chromosome pairs and walks toward microtubule plus ends.
"It's like trains on a track. The chromosomes are being shuttled along these lateral bundles to the center of the spindle," she said.
Further studies have indicated other proteins in the same complex as KLP-19. Currently, a part of the Wignall lab focuses on projects that set out to understand how this ring complex forms, mediates the movements and segregates chromosomes.
"Her speaking style was clear and crisp. You can tell why she won teaching awards," Trina Schroer, seminar host and a professor in the Department of Biology, said in an interview with The News-Letter. "Anybody who is a biology major learns about mitosis and the way mitotic spindles form, where there is a known mechanism for focusing microtubules together. In meiosis, uniformly, they do not use that mechanism."
Schroer particularly appreciated Wignall’s proposal that oocyte meiosis is acentrosomal due to it being evolution's solution to limiting centrosome numbers. Schroer encourages all undergraduates to attend the Department of Biology seminars.
"Our goal is to bring in science and scientists that are doing great work that is of interest to the department yet distinct from what we do ... and interesting to a wide variety of perspectives," she explained.