Learning and memory comprise a fundamental part of our lives, allowing us to keep up with changes in the environment and acquire new information about the world. It is well established that a brain region known as the hippocampus is important for such abilities. However, the mechanism governing hippocampal-dependent cognitive function remains elusive. Recently published in Neuron, a study has shown that the hippocampus retrieves memory by activating networks in the cerebral cortex, providing an insight into the biological basis of learning and memory.
The story of the hippocampus began with Henry Molaison, more famously known as “HM”. HM suffered from epilepsy, a disorder characterized by recurrent spontaneous seizures. Since his physician determined that the seizures were arising from the temporal lobes, HM underwent a surgery to remove parts of this area of the brain, including the hippocampus. Although HM no longer had seizures, he developed retrograde and anterograde amnesia. He could not remember events before the surgery, nor could he form new memories. As a result, scientists realized that the hippocampus was the learning and memory region.
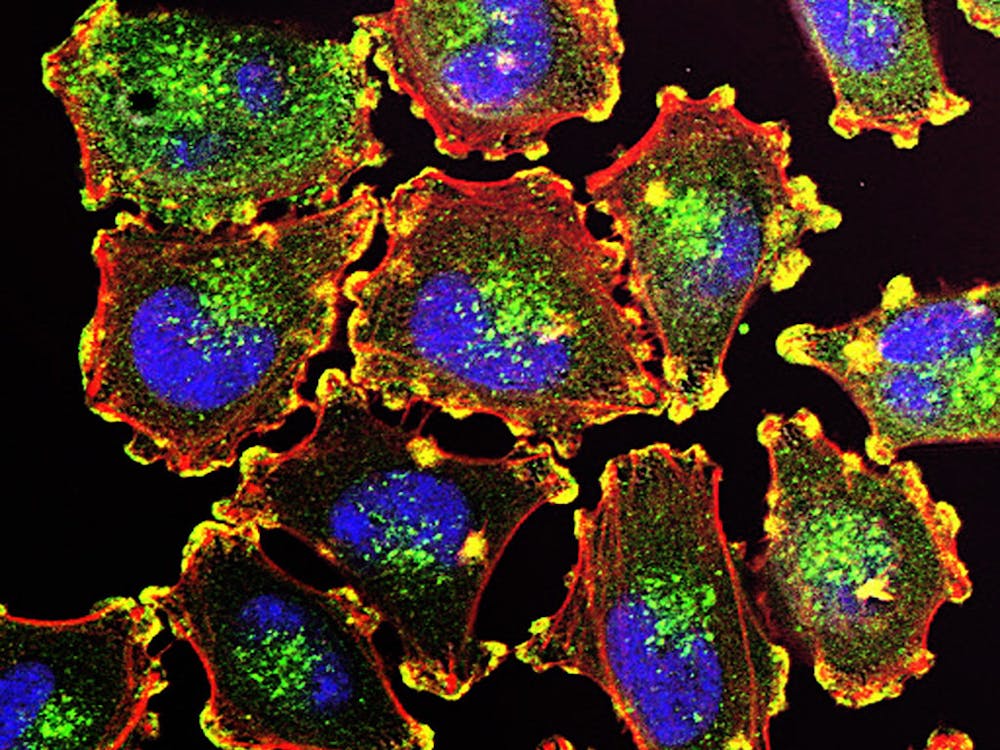
How does the hippocampus drive learning and memory? It is believed that stimuli from the external world leave behind physical traces in the brain known as “engrams”. By reactivating engram-bearing neurons that code for specific stimuli, the brain can perform memory recall. Although learning and memory are primarily associated with the hippocampus, some researchers have suggested that engram cells may be in the cortex as well. Thus, learning and memory are not localized to a single region of the brain but rather encompass a widely distributed hippocampal-cortical network. However, how the hippocampus interacts with the cortex to drive memory functions remains unclear.
Given that the cortex is responsible for conscious experience, it is possible that the conscious element of memories is stored in cortical engram neurons rather than in the hippocampus. Thus, it has been hypothesized that the hippocampus activates cortical neurons to drive conscious recall of memories.
Combining innovative genetic methods with optogenetics, Brian Wiltgen, an associate professor in the department of psychology at UC Davis, and his colleagues tested this popular hypothesis by functionally perturbing hippocampal neurons in mice. First, they placed the mice in cages where electrical shocks are delivered to their feet. Since the mice will learn to remember that a specific cage is associated with painful shocks, they will “freeze” in fear when they are returned to the original cage.
However, the mice are not just normal mice. As memories of the cages are formed, certain neurons activate in order to encode those memories, becoming engram cells. These mice have been genetically engineered so that engram cells are labeled with specialized molecules, allowing scientists to both visualize the engram neurons and deactivate them by shining a light.
As expected, Wiltgen found the presence of engram cells throughout the hippocampus and cortex after the mice had been trained in context-dependent fear conditions. When the hippocampal engram cells were silenced with light, the scientists made two intriguing discoveries. First, the mice were less likely to freeze in the cages, suggesting that they had “forgotten” their past experience of being foot shocked. Secondly, turning off hippocampal neurons also deactivated engram neurons in the cortex. Thus, conscious memory recall of past events requires activation of engram cortical neurons by the hippocampus.
This interesting study reveals important insights into how different regions of the brain interact to drive memory recall. However, I do have to emphasize an important key point. I have seen popular science news sites such as Science Daily report this study by claiming that scientists erased specific memories with light. This is entirely inaccurate. The scientists simply blocked the activity of engram cells, which remained fully intact. Thus, the memory is still stored inside these engram cells, and silencing these neurons prevented the mice from recalling the memory. In order to erase the memory, the scientists would have to kill off the cells or remove them from the brain.
Nevertheless, the study is still an important step towards understanding the physical nature of memories by confirming an old hypothesis in the field. With a greater insight into the biology of learning and memory, we may be able to develop better treatments for memory disorders such as Alzheimer’s Disease.
There are a wide variety of diseases that impair the growth of the brain and nervous system, ranging from autism spectrum disorders to schizophrenia. With this large number of disorders comes an even larger number of treatments, from medications to therapies to surgeries. However, many of these seemingly different neurodevelopmental disorders may share a common cause. One treatment could be developed that would be effective for many different disorders.
Previous challenges in studying the genetic causes of neurodevelopmental disorders have resulted from the difficulty in pinpointing which cellular functions are affected by which genes. Mutations in a single gene can cause separate disorders with notably different symptoms; for example, the same deletion on a segment of chromosome 15 can cause either Angelman syndrome or Prader-Willi syndrome, depending on whether the maternal or the paternal chromosome is affected.
On the other hand, a single “umbrella” disorder can originate from a complex variety of genetic mutations and molecular changes. This is the case with Alzheimer’s disease, for which the familial variant has been linked with mutations in no fewer than three different chromosomes.
A study at McGill University, led by Carl Ernst and published in the American Journal of Human Genetics, has proposed that certain genetic mutations can have remarkably similar effects on the growth and development of the brain and central nervous system.
Ernst and his team focused specifically on two enzymes: transcription factor 4 (TCF4) and euchromatic histone methyltransferase 1 (EHMT1). When these enzymes are affected, their altered functions lead to two different autism spectrum disorders.
The two disorders, named 18q21 deletion syndrome and 9q34 deletion syndrome, lead to similar delays in intellectual development and correspond with increases in psychiatric conditions such as depression and anxiety. Nevertheless, the disorders have separate names because of differences in the physical characteristics of affected individuals, their frequency of occurrence and, most importantly, the chromosomes on which the mutations occur.
The researchers altered the activity of each enzyme in human fetal brain cells, which are undifferentiated. This means that all of their genes are still expressed, and as a result, the cells are less specialized. During normal development, cells become differentiated for more specific functions, such as fighting infections or transferring energy.
Changes in either TCF4 or EHMT1 both resulted in similar changes in molecular activity in the fetal cells. These changes resembled those expressed by more mature brain cells, suggesting that the deficiencies in neurodevelopmental disorders result from these cells’ attempts to differentiate prematurely.
The research points to the fact that seemingly different disorders induced by different genetic mutations can cause similar molecular changes within cells. This could allow for a more general classification of neurodevelopmental disorders based on the molecular functions that are affected. While the possibility of such a classification could greatly simplify the ways in which scientists can understand how genetics affect human development, it is only the first step in understanding why the final effects of neurodevelopmental disorders can be so diverse.




















Please note All comments are eligible for publication in The News-Letter.